Pharma:
Electronic Batch Records
Transform Your Data Management: The Efficacy of Electronic Batch Records
Electronic batch records (EBRs) play a critical role in the pharmaceutical manufacturing process. They are used to document and manage all critical steps involved in the production of a batch of drugs, from the receipt of raw materials to the final product.
In the context of Pharma 4.0, EBRs can be improved by incorporating advanced technologies and tools, which can provide real-time data and information to optimize the manufacturing process.
Some questions to ask before starting:
- Is our batch record fully digitized?
- Can they be reviewed agilely? (Review by exception).
- My electronic records will be within regulations and will meet all Data Integrity requirements.
Digitize your records from start to finish
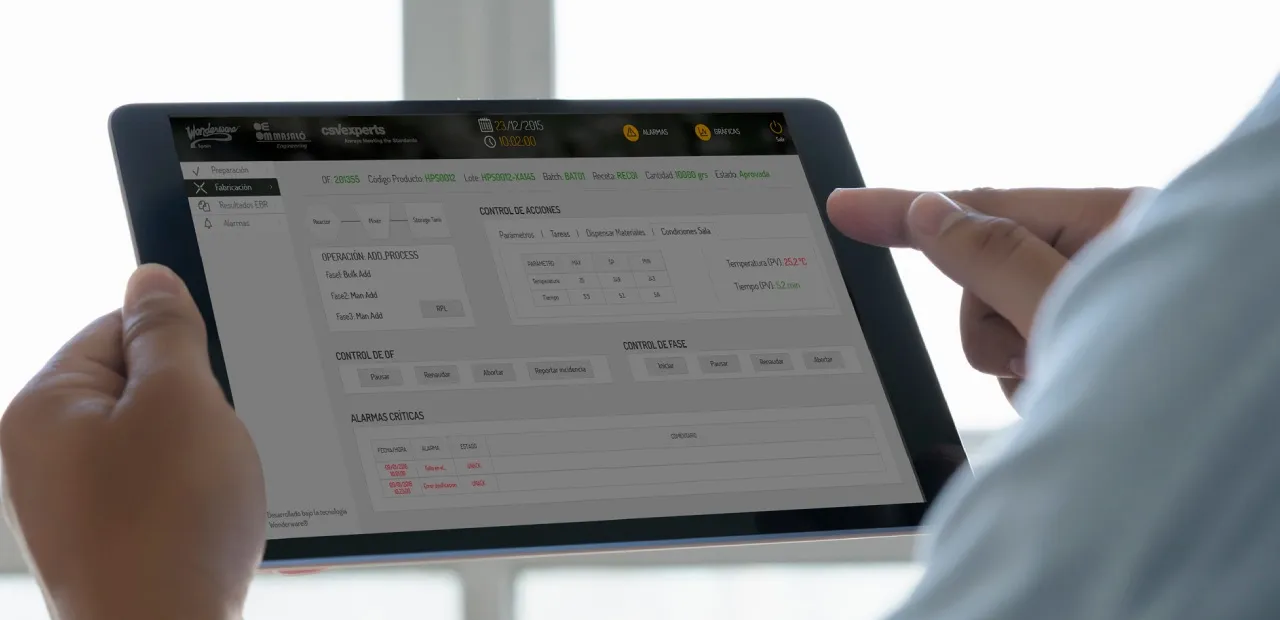
Is our Batch Record Fully Digitized?
By deploying different parts of a guided manufacturing system, the recording of all kinds of data and events related to regulated operations is enabled. You can integrate data from different repositories for later consultation or exploitation.
Exploit in an Integrated Way.
- Data related to environmental hygiene.
- Data related to Weight and preparation of raw materials.
- Data related to the different steps of Batch manufacturing.
- Data related to In Process Control (IPC).
- Data related to dispensing.
- Data related to primary conditioning.

Improve Efficiency in Reviewing your EBR Data
Can They be Reviewed Agilely? (Review by Exception).
Every day, all activities related to the manufacture and quality control of pharmaceutical products generate and rely on a huge amount of data, some of specifications and others that must be collected systematically.
All data collected must be properly treated to become information.
A management not only effective, but also efficient can improve the productivity of operations.
A Digital Transformation Process
- Save time and avoid errors, review only the records that require your attention. The system allows you to show them all or only those marked for the Review By Exception.
- Improve your situational awareness, receiving alerts whenever a record that requires your attention is generated. Detecting situations that lead to non-conformities in the earliest possible stage can make a difference.
- Integrate calculations and formulas into your records. Avoid potential errors and inefficiencies by doing it in an integrated way.
Consult all the data on a single platform. Navigate through the data and turn it into information.

ALCOA + your Records beyond Data Integrity
Can They be Reviewed Agilely? (Review by Exception).
The correct treatment of data is crucial to avoid risks that may affect the quality of products, and therefore patient safety.
Following the ALCOA Principle, your Data Will Meet the Needs of Data Integrity:
Your data will be Attributable because data and metadata are recorded in a contextualized way while the different procedures of guided manufacturing are executed. Any event or planned relationship will be recorded.
The readability of the data is guaranteed as they are electronic records.
The data is recorded in real time as the different phases of the production or quality processes are executed. The original recorded data is marked, so that any additional data recorded is easily distinguishable and requires an Audit Trail. All data will be electronically signed. Access to the data is secure and operators cannot change the data collected automatically.
The original recorded data is marked, so that any additional data recorded is easily distinguishable and requires an Audit Trail. All data will be electronically signed. Access to the data is secure and operators cannot change the data collected automatically.
The accuracy of the data acquired automatically in time.
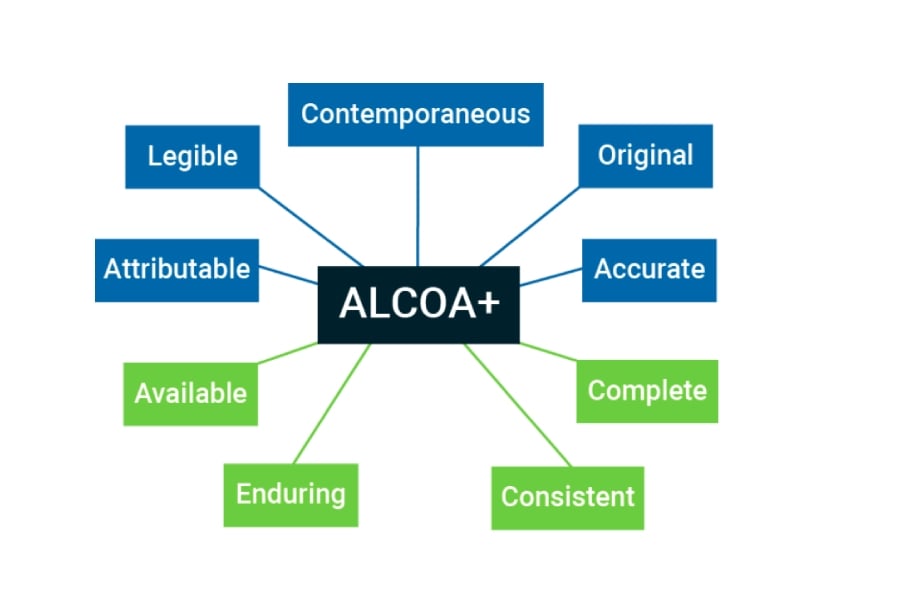
But You Can also Benefit from other Features that Will Take your Management to another Level:
- All data will be quickly available for consultation throughout the life of the record.
- The data will be recorded on durable media, which allow its safeguarding and consultation. All data will be recorded together with its date and time stamp and in the order that facilitates its consultation and analysis
- All data will be recorded together with its date and time stamp and in the order that facilitates its consultation and analysis
- The system will be complete as it will include all data relating to repetitions or reanalysis
Get in Touch with an Expert
Fill in your information and we’ll contact you to handle your request.